Impact-R to Determine Effect of Erythrocytosis and Thrombocytosis
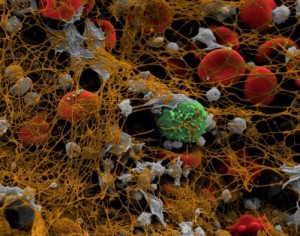
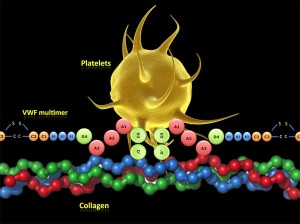
Wednesday, January 18th, 2012Impact-R, a cone and platelet analyzer can be used to determine the effect of increased erythrocyte and thrombocyte counts on platelet adhesion and aggregation.
In a study by Peerschke, et.al., the cone and platelet analyzer was used to determine platelet adhesion in terms of surface coverage (%SC) and platelet aggregation in terms of aggregate size (AS in um).
The study noted that erythrocytosis increased aggregate size and thrombocytosis increased surface coverage. The specimen used was whole blood from healthy individuals with varying RBC and platelet counts.
The study also investigated blood from individuals with myeloproliferative diseases. They noted that differences in the platelet function parameters were seen in MPD patients undergoing different therapies.